Kevin Drisko was at CSI Devices for Heart Failure (DHF) in Frankfurt

Kevin Drisko recently represented the HEU-EFS project at the CSI Devices for Heart Failure (DHF) conference in Frankfurt, presenting during the session titled “Time for the Pilots: Towards a New Harmonized Approach to EFS in Europe.”
The event provided an important platform to share the latest progress of the HEU-EFS initiative. Kevin highlighted that the pilot phase is about to begin, marking a major milestone for the project. These pilots will test the newly developed methodology through live use cases and are open to both consortium members and the wider public.
It was a productive and engaging week, filled with valuable discussions around Early Feasibility Studies (EFS) and innovation in heart failure technologies. The strong collaboration across partners has been instrumental in bringing the project to this exciting stage.
To cap off the conference, Kevin was honored with the award for Best Poster Presentation, recognizing both the quality of the work and the impact of the HEU-EFS project within the community.
ISPOR US 2025
At ISPOR US 2025, two sessions addressed critical aspects of pre‑market clinical research for medical devices, with a focus on Early Feasibility Studies (EFS) and the challenges associated with conducting early‑stage investigations in Europe and internationally.
Challenges of Pre‑Market Clinical Investigations of Medical Devices: A Multi‑Stakeholder Perspective
Oral Presentation
Moderator: Giuditta Callea
Speakers: Rosanna Tarricone, Carlo Federici, Helen Banks, Maria Luisa Buzelli, Francesco Benito Malandrini, Franco Luigi Zurlo, Marta Kerstan, Monica Tocchi, Nicolas Martelli, Tess Martin, Ornella Tangila Kayembe, Stephane Piat, Laura Sampietro‑Colom, Andrea Rappagliosi, Claudia Louati, Yasemin Zeisl, Daniel Beltran, Adrián Valledor, Marta Bragagnolo, Marit Erna Austeng, Alexandra Herborg Cornelius Poulsson, Kristian Kidholm, Lise Kvistgaard Jensen, Benedetta Brancadoro, Carmen Furno, Sebastian Kuhn.
This session examined the challenges and barriers involved in pre‑market clinical investigations (CIs) of medical devices and provided evidence to support the development of a harmonized European Early Feasibility Studies (EFS) Program.

Objectives
To gather perspectives from multiple stakeholders—including regulators, HTA bodies, clinical sites, notified bodies, patient associations, and technology developers—to inform future EFS policy and operational frameworks in the EU.
Methods
The work presented included:
- an online survey for technology developers,
- open‑ended interviews with stakeholders across Europe,
- and a focus group with patient advisory group members and patient associations.
Key Findings
- The EU is viewed as a preferred location for pre‑market CIs due to expertise, site capabilities, and timelines.
- A major barrier identified was limited dialogue across stakeholders, contributing to complexity and delays.
- Challenges also included risk–benefit evaluation, device risk assessment, study design, endpoints, enrolment targets, and a lack of clear templates or guidance.
- Patients identified fragmented information and insufficient time to evaluate participation risks as significant issues.
Conclusions
Stakeholders highlighted the multifaceted hurdles in bringing medical device innovations to market. Strengthening early interaction among all parties involved was identified as essential to improving clarity, reducing delays, and supporting a more efficient environment for pre‑market clinical investigations.
Bridging the Gap in Early‑Stage Clinical Research: Lessons for EU Stakeholders from the US FDA Early Feasibility Studies Program
Moderator: Giuditta Callea
Speakers: Dorothy Abel, Kevin Drisko
This session examined what the EU and other jurisdictions can learn from the U.S. FDA Early Feasibility Studies (EFS) Program, established to support innovative, significant‑risk medical devices when non‑clinical testing is insufficient.
Topics Addressed
- the purpose and characteristics of early‑stage clinical investigations for high‑risk devices;
- challenges faced when non‑clinical testing cannot fully inform device safety or performance;
- insights from the first decade of the FDA EFS Program, including best practices and oversight mechanisms;
- implications of Europe’s fragmented pre‑market regulatory landscape for conducting EFS;
- perspectives from regulators, academia, industry, clinical stakeholders, and findings from the HEU‑EFS project.
The session concluded with a moderated roundtable addressing audience questions, engaging stakeholders such as regulators, healthcare providers, patient associations, CROs, researchers, and technology developers.
Overall Event Perspective
Together, these two sessions demonstrated the growing relevance of Early Feasibility Studies in supporting responsible medical device innovation and highlighted the importance of harmonized frameworks, stakeholder collaboration, and evidence‑based policy development in both the EU and the U.S.
Italian Health Economics Association (AIES) XXIX Annual Conference
Setting Up a Harmonized Framework for Early Feasibility Studies in the European Union: What Value(s)?
Italian Health Economics Association (AIES) XXIX Annual Conference

At the XXIX Annual Conference of the Italian Health Economics Association (AIES), Session VIII focused on “Setting Up a Harmonized Framework for Early Feasibility Studies in the European Union: What Value(s)?”. The session brought together several speakers to discuss regulatory, methodological, and practical aspects of Early Feasibility Studies (EFS) for medical devices.
Chair: Giuditta Callea
Speakers included:
• Helen Banks – “Pre-market clinical investigations of medical devices: European regulatory framework and international standards”
• Maria Luisa Buzelli – “Challenges and barriers to pre-market clinical investigations of medical devices: a systematic literature review”
• Francesco Malandrini – “Pre-market approval pathways for clinical investigations of medical devices: a global perspective”
• Franco Luigi Zurlo – “State of the art of Early Feasibility Studies for medical devices”
The session provided insights into the current landscape of pre‑market clinical investigations, existing challenges, and the evolving approach to harmonizing Early Feasibility Studies within the European Union.
HTAi 2024 Annual Meeting
Oral presentation at the HTAi 2024 Annual Meeting, Spain
Presenter: Adrian Valledor
At the HTAi 2024 Annual Meeting in Spain, Adrian Valledor delivered an oral presentation titled “Harmonized Approach for Medical Devices in the EU to EFS” to the Medical Devices Interest Group. He represented Fundació Clínic per a la Recerca Biomèdica, Institut d’Investigacions Biomèdiques August Pi i Sunyer (IDIBAPS), and Hospital Clínic de Barcelona.
The presentation provided an overview of the HEU‑EFS project and contributed to the ongoing exchange of knowledge within the health technology assessment community.
Acknowledgements were extended to Laura Sampietro‑Colom for enabling the participation, as well as to colleagues Paula Closa Granados, Sonia Muñoz López, PhD, Marcelo Soto Alarcón, and Joan Fibla Reixachs for their support during the session. Additional appreciation was given to Daniel Beltran Argudo for his continued guidance and contributions.

MedTech Forum 2024
Learn from the best: some IHI winners sharing their experiences
Parallel session at MedTech Forum 2024, Vienna
Speaker: Fanny van der Loo
This parallel session took place at MedTech Forum 2024 in Vienna. The forum is organized in the context of MedTech Europe, the European trade association for the medical technology industry, including diagnostics, medical devices, and digital health.

Patient Engagement Open Forum (PEOF) Provocative Co-creation
Patients at the Heart of Early MedTech Development
At the Patient Engagement Open Forum (PEOF) 2024 in Italy, the parallel session “Patients at the Heart of Early MedTech Development” provided a structured overview of how patient involvement can be integrated into Early Feasibility Studies (EFS) for medical devices. The session combined expert presentations with facilitated group discussions to examine expectations, information needs, and decision-making considerations from a patient perspective.
Speakers and facilitators included:
• Francesco Malandrini, SDA Bocconi School of Management
• Claudia Louati, European Patients’ Forum (EPF)
• Yasemin Zeisl, EPF
• Valentina Strammiello, EPF
• Phil Collis, HEU‑EFS Patient Advisory Group
• Lucia Feito Allonca, HEU‑EFS Patient Advisory Group
Topics addressed during the session included:
- the purpose and structure of Early Feasibility Studies,
- opportunities to integrate patient input at an early stage,
- challenges related to communication, risk understanding, and informed decision-making,
- and practical considerations for multi-stakeholder collaboration in early MedTech development.
Key conclusion: patient involvement in the early phases of device development improves the relevance, quality, and acceptability of innovative medical technologies.
Thank you to all contributors and participants for advancing the discussion on patient‑centred approaches within the HEU‑EFS initiative.

ESC CRT Roundtable
Industry Perspective on the Evolution of EU Medical Device Regulation: Insights from Brussels
On 17–18 April 2024, Brussels hosted the plenary meeting “Priorities for the Evolution of Medical Device Regulatory Approval Systems,” bringing together key stakeholders to discuss the future of medical device regulation in Europe. The two-day event focused on critical objectives, including:
- Harmonization of global medical device regulations
- Opportunities for priority access to health technology innovation
- Regulatory considerations for orphan devices
This meeting served as a follow-up to a previous event held in November 2022, continuing the dialogue on how regulatory frameworks can better support innovation while safeguarding patient safety.

Industry Contribution
Andrea Rappagliosi from Edwards Lifesciences delivered an oral presentation offering an industry perspective on the EU medical device regulatory system and how it should evolve. His insights emphasized the need for streamlined processes that enable faster access to innovative technologies without compromising compliance and patient safety.
Why This Matters for HEU-EFS
The HEU-EFS Project is deeply aligned with these goals. By promoting a harmonized approach to Early Feasibility Studies (EFS) for medical devices, the project seeks to integrate patient perspectives into the design and evaluation of future cardiovascular therapies. This ensures that innovation translates into meaningful patient benefit, while supporting a lifecycle approach to clinical evidence generation.
The CRT meeting provided a powerful platform to showcase this vision and engage with regulators, industry leaders, and healthcare stakeholders shaping the future of cardiovascular regulation in Europe.
Events like this highlight the importance of collaboration between regulators, industry, and research organizations to create a regulatory environment that fosters innovation and improves patient outcomes.
CSI Devices for Heart Failure (DHF)
Kevin Drisko from Edwards Lifesciences had the opportunity to close the CSI Congress Devices for Heart Failure meeting on 09 December 2023 with a presentation titled “Harmonized Approach to Early Feasibility Studies (EFS) for Medical Devices in the EU.” This Innovative Health Initiative (IHI)-funded activity was enthusiastically received by attendees committed to accelerating and advancing device-based therapies for heart failure.
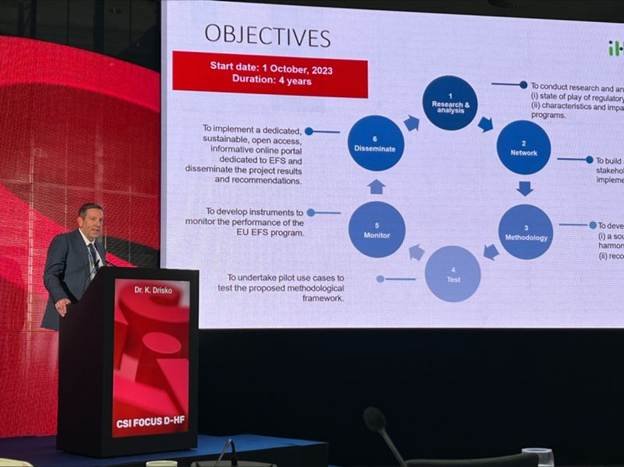
Special thanks go to the meeting organizers for including this important topic in the agenda, and to William Abraham and Horst Sievert for moderating the session. Appreciation is also extended to Giuditta Callea and Andrea Rappagliosi for their leadership in the #HEUEFS project.
ISPOR 2023
Copenhagen, Denmark
Session Title: Setting up a Harmonized Methodology to Promote Uptake of Early Feasibility Studies for Clinical and Innovation Excellence in the European Union: What Value(s)?
Why This Matters
The European Union currently lacks standardized procedural frameworks for the uptake of Early Feasibility Studies (EFS). This gap creates significant challenges for applying a lifecycle approach to clinical evidence generation for medical devices. As a result, patient access to innovative technologies is delayed, and EU competitiveness suffers.
To address these issues, the HEU-EFS Project, a 4-year initiative funded by the Innovative Health Initiative (IHI) and co-funded by six industry partners, aims to establish a harmonized approach to EFS for medical devices across Europe.

Giuditta Callea represented Università Bocconi, the leader of the HEU‑EFS Consortium, presenting the project’s rationale and objectives. She also moderated an engaging discussion on the potential of a future EU‑wide EFS Program with:
- Melvin Tom – Trinity College Dublin
- Kristian Kidholm – Center for Innovative Medical Technologies, OUH Odense Universitetshospital – Svendborg Sygehus
- Fanny van der Loo – Edwards Lifesciences
This session was designed for:
• EU and national regulators
• Competent authorities & notified bodies
• HTA bodies
• Healthcare providers
• Patient organizations
• Research organizations
• Legal & ethical experts
• CROs
• Health technology developers
LIVE Discussion on Early Feasibility Studies & the HEU‑EFS Project
Organised by TrendSanità
TrendSanità recently organized a LIVE discussion dedicated to the growing role of Early Feasibility Studies (EFS) in advancing medical device innovation across Europe. The session featured Giuditta Callea, coordinator of the Horizon Europe–funded HEU‑EFS project, who contributed her expertise on how EFS can support fast yet safe pathways from laboratory development to real‑world patient access.
During the event, participants:
- Explored the objectives and latest outcomes of the HEU‑EFS project, including recommendations, templates, and metrics developed by 22 international partners
- Discussed how EFS strengthen early clinical evidence in alignment with ISO 14155 and EU regulatory requirements
- Reviewed the project’s achievements and looked at future directions for harmonising EFS across the EU
- Reflected on the strategic value of EFS for innovation, patient access, and the competitiveness of the European medical device sector
This LIVE session offered professionals from industry, clinical research, patient organizations, and regulatory fields an opportunity to gain clarity on the EFS landscape and engage with experts shaping its evolution.
Speakers:
- Giuditta Callea – SDA Bocconi School of Management, Principal Investigator HEU‑EFS
- Marta Bragagnolo – Global Heart Hub, HEU‑EFS Partner
- Guido Beccagutti – Director General, Confindustria Dispositivi Medici
Host:
Rossella Iannone, Editor‑in‑Chief, TrendSanità
See the full event here: https://trendsanita.it/dispositivi-medici-cosa-sono-gli-early-feasibility-studies/
