OPEN CALL FOR PILOTS
Early Feasibility Studies in European Union
ISO 14155:2020 defines Early Feasibility Studies (EFS) as limited clinical investigations designed to evaluate the initial clinical safety and performance of a medical device.
Conducted at an early stage, before the device design is finalised, EFS help collect critical insights on a device’s design, safety, functionality, and performance, especially when further nonclinical testing is unavailable or not informative.
Why EFS Matter
EFS play a key role in the evidence generation pathway, offering the potential to:
- Increase investment attractiveness in the European medical technology landscape
- Accelerate patient access to safe and innovative medical devices.
- Strengthen the EU’s position as a hub of clinical excellence and innovation.
The Current Challenge
While EFS are permitted under the EU Medical Device Regulation (MDR), there is currently no harmonised pathway or specific framework to support their systematic adoption across the European Union (EU).
The HEU-EFS initiative aims to change this by developing a unified, structured approach to Early Feasibility Studies, enabling innovation and collaboration across Europe.
The HEU-EFS project
HEU-EFS is a four-year European project called Harmonised Approach to Early Feasibility Studies for Medical Devices in the European Union, which started in October 2023 and involves 22 public and private organisations across Europe.
The project is co-funded by the Innovative Health Initiative (IHI) under the Horizon Europe Framework Programme and 6 industry partners for a total budget of €19 million. The overall goal of the initiative is to develop a harmonised methodology and enhance the uptake of EFS in the EU. For more information, please visit www.heuefs.eu.
Call for expression of interest
HEU-EFS consortium is pleased to announce the launch of an open call for pilots to validate the methodological framework of the harmonised approach to EFS for medical devices in the EU.
As a first step, under this call, pilot proposers are invited to submit Expression of Interest online form to indicate their interest to conduct an EFS pilot study using methodology developed by the HEU-EFS consortium.
The pilot will provide innovators with a unique opportunity to take their innovations via a pilot structured EFS regulatory process. Particularly, the participants will:
- Obtain guidelines and templates for EFS submissions along with structured early engagement with the National Competent Authorities (NCAs).
- Benefit from a unique learning opportunity and engage with our consortium experts.
- Contribute feedback that will influence the development of the future EU EFS programme.
Who can apply and eligibility conditions
Sponsors wishing to conduct pilot EFS clinical investigations in Europe who meet the following criteria:
1. The clinical investigation must be consistent with the definition of Early Feasibility Study as per ISO 14155:2020.1
2. They must be a high-risk devices (Class III and Class IIb), where a clinical investigation will be required as part of the conformity assessment.
3. They must meet at least one of the following criteria:
- Breakthrough Device/Unmet Need – utilized to collect early clinical data when no equivalent device exists.
- Anatomical understanding – utilisation of the EFS to better understand physiology and anatomical limitations of the device under development.
- New /Expanded intended uses or indication for use – the application of an existing/approved device in a new patient population or application
- They must request approval in at least one European country (i.e. countries within the EU or European Economic Area).
Submission of the call of interest
Applications should be submitted by completing and sending the online form no later than March 31, 2026.
The data provided will be handled as strictly confidential. It will be stored by Bocconi University (project coordinator) and accessible to members of the Screening Committee.
For any questions regarding the Open call for pilots, please contact the HEU-EFS Point of Contact at pilot@heuefs.eu.
Process
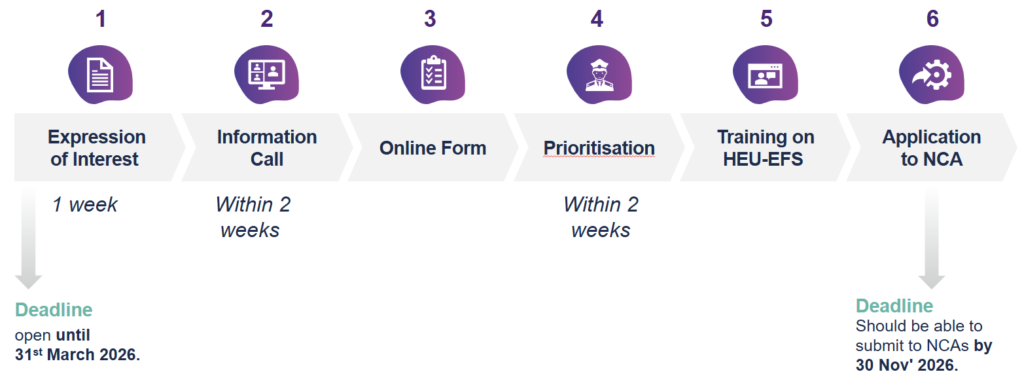
The Expressions of Interest will be assessed by the Screening Committee, coordinated by Bocconi University to review matching of the pilots with the scope of the methodology framework and proposer’s readiness.
Proposers and members of the Screening Committee will meet online (30-minute) and discuss the expressed interest.
After positive assessment, proposers will be provided with the list of next steps and with a link for submitting a self-evaluation checklist via the online system QualtricsXM provided by Bocconi University.
Once an applicant is selected for the pilot, the Point of Contact will make available an information package and training outlining the next steps and providing instructions and templates. No services will be provided on the conduct of the clinical trial implementation. Proposers should finance their own studies. No public funding will be available from Innovative Health Initiative or from HEU-EFS consortium.
Learn more
Resources
Find all project deliverables here.
HEU-EFS at the 33rd Annual EORS Meeting
A Showcase of Innovation and Collaboration


We are excited to share the highlights from the 33rd Annual Meeting of the European Orthopaedic Research Society (EORS) in Davos, Switzerland, where the HEU-EFS project was prominently featured.
The event, held from June 16 to 19, 2025, brought together leading experts and stakeholders to discuss critical systems, infrastructure, and policy frameworks needed to drive innovation in orthopaedic research. The HEU-EFS project used this platform to disseminate its latest results and developments through a panel discussion.
Here are some reflections from our esteemed panelists:
- Marta Kerstan, DePuy Synthes: “The symposium was a resounding success! The lectures provided invaluable perspectives from patient organizations, medical devices and digital health technology, surgeons, regulators, scientists, and investors. The interactive panel discussion fostered idea exchanges, making it a remarkable opportunity for learning and collaboration.”
- Yasemin Zeisl, EPF: “Engaging patients throughout the lifecycle of medical devices is essential. It enhances legitimacy, transparency, and accountability in clinical research and innovation, ensuring that real-world experiences shape their development.”
- Susan Partridge, BSI: “This was a great opportunity to present the Notified Body perspective on early feasibility studies at the HEU-EFS symposium in Davos. I thoroughly enjoyed the valuable insights from all panel members.”
- Alexandra Poulsson, NIPH: “The EORS combined conference with ARI Orthopaedics and the International Society for Computer Assisted Orthopaedic Surgery (CAOS) was a great opportunity to showcase the achievements of the HEU-EFS project. It fostered important discussions with researchers, clinicians, patient associations, Notified Bodies, SMEs, and large medical device producers.”
- Ana Matos Machado, TUV Sud: “Harmonizing EFS can significantly enhance the EU market’s attractiveness for innovation”.
- Marta Bragagnolo, GHH: “Involving patients early in the process leads to better outcomes for everyone—patients, clinicians, and innovators alike. Innovation without patient partnership is incomplete. Let’s build healthcare solutions with patients, not just for them.”
We are proud of the impact and visibility the HEU-EFS project has gained through this event and look forward to continuing our efforts to drive innovation in clinical trials.
Innovative Health Initiative (IHI) MedTech Europe EFPIA – European Federation of Pharmaceutical Industries and Associations COCIR EuropaBio – the European Association for Bioindustries Vaccines Europe
#IHITransformingHealth #EvidenceGeneration #MedTech #ClinicalTrials #OrthopaedicResearch







Featured in the photos (l-r): Alexandra Poulsson from the Norwegian Institute of Public Health (podium) and Marta Kerstan from DePuy Synthes, Thomas Randau from Krankenhaus der Augustinerinnen, Marta Bragagnolo from Global Heart Hub, Yasemin Zeisl from European Patients’ Forum, Ana Matos Machado from TÜV SÜD, Sebastian Kuhn from The Philipp University of Marburg, Susan Partridge from BSI, and Roland Herzog from AO Foundation
HEU-EFS: Advancing Inclusive Clinical Trials for Medical Devices

Developing and validating a medical device takes years of research and significant investment. #ClinicalTrials are a critical part of this journey, making it possible to bring life-changing treatments and technologies to patients. They offer patients early access to potentially groundbreaking therapies and play a vital role in advancing medical knowledge, improving care, and saving lives. 𝗛𝗘𝗨-𝗘𝗙𝗦: 𝗔𝗱𝘃𝗮𝗻𝗰𝗶𝗻𝗴 𝗜𝗻𝗰𝗹𝘂𝘀𝗶𝘃𝗲 𝗖𝗹𝗶𝗻𝗶𝗰𝗮𝗹 𝗧𝗿𝗶𝗮𝗹𝘀 𝗳𝗼𝗿 𝗠𝗲𝗱𝗶𝗰𝗮𝗹 𝗗𝗲𝘃𝗶𝗰𝗲𝘀
On the occasion of International Clinical Trials Day 2025, we honour the patients, clinicians, researchers, scientists and regulators across Europe who make clinical research possible. 👏
At the HEU-EFS project, we contribute to a better ecosystem for Early Feasibility Studies (#EFS) for medical devices in the EU. EFS are a critical step in the early #EvidenceGeneration pathway. By working on a Harmonised Approach to EFS in the EU (HEU-EFS), we aim to make Europe a more attractive environment for medical innovation and ensure patients have timely access to cutting-edge breakthrough technologies.
Innovative Health Initiative (IHI) MedTech Europe EFPIA – European Federation of Pharmaceutical Industries and Associations COCIR EuropaBio – the European Association for Bioindustries Vaccines Europe
#ICTD25 #MedTech #PatientCare
HEU-EFS at RAPS Euro Convergence 2025
The HEU-EFS consortium participated in RAPS Euro Convergence 2025, held from 13–16 May at the SQUARE Brussels Meeting Centre in Brussels, Belgium. This premier event brought together regulatory professionals, industry leaders, and innovators to explore the evolving landscape of healthcare regulations and medical technologies.

As part of the conference, a poster titled “Are Early Feasibility Studies (EFS) an Orphan Approach in the Regulatory Pipeline for Innovative Medical Devices?” was presented by members of the HEU-EFS project team, including Adrián Valledor Sánchez, in collaboration with Daniel Beltran Argudo and Laura Sampietro-Colom.
The poster explored the underutilization of Early Feasibility Studies (EFS) in Europe and their potential to accelerate innovation in the medical device sector. It emphasized the need for a harmonized regulatory framework to support EFS implementation, aligning with the broader goals of the HEU-EFS project—a collaborative initiative under the Innovative Health Initiative (IHI).
Throughout the event, the HEU-EFS team represented by Fundació Clínic per a la Recerca Biomèdica, Institut d’Investigacions Biomèdiques August Pi i Sunyer (IDIBAPS), and Hospital Clínic de Barcelona, engaged with stakeholders across the regulatory and MedTech communities, fostering dialogue on how EFS can bridge the gap between early innovation and patient access. The discussions underscored the importance of integrating patient perspectives and real-world evidence into early-stage research and regulatory planning.
RAPS Euro Convergence 2025 served as a vital platform for the HEU-EFS consortium to share insights, gather feedback, and strengthen partnerships aimed at transforming the future of medical device development in Europe
Tags: RAPS2025, HEUEFS, RegulatoryAffairs, MedicalDevices, EarlyFeasibilityStudies, InnovationForPatients, HealthTech
HEU-EFS @ ISPOR 2025
Advancing Early Feasibility Studies for Medical Devices
The HEU-EFS consortium was honoured to participate in ISPOR 2025, held from May 13–16 in Montréal, Canada—the leading global conference for Health Economics and Outcomes Research (HEOR). This year’s theme, “Collaborating to Improve Healthcare Decision Making for All: Expanding HEOR Horizons,” emphasized the importance of cross-sector collaboration in shaping equitable, data-driven healthcare systems

As part of the conference’s dynamic Issue Panel sessions, the HEU-EFS team presented “Bridging the Gap in Early Stage Clinical Research: Lessons for EU Stakeholders from the US FDA Early Feasibility Studies Program“
Moderated by Giuditta Callea (SDA Bocconi), the panel featured expert insights from:
- Dorothy Abel, Abel & Wolf Consulting
- Kevin Drisko, Edwards Lifesciences
The session explored the structure and impact of the US FDA’s Early Feasibility Studies (EFS) Program, highlighting its role in accelerating medical device innovation. Panelists discussed how similar strategies could be adapted within the EU regulatory landscape to address fragmentation and support early-stage clinical research.
Representing a collaboration of 22 public and private partners, the HEU-EFS project aims to develop a harmonized framework for EFS across Europe. The panel emphasized the importance of regulatory alignment, patient-centered design, and international knowledge exchange to foster innovation and improve patient access to novel technologies.
Why It Mattered
The HEU-EFS initiative aligns closely with ISPOR’s mission to make HEOR insights more actionable and inclusive. By integrating EFS into the broader conversation on evidence generation and value-based healthcare, the consortium contributes to shaping a more agile and responsive regulatory environment for medical devices.
Tags: ISPOR2025, HEUEFS, EarlyFeasibilityStudies, MedicalDevices, RegulatoryAffairs, EvidenceGeneration, InnovationForPatients, HealthTech
MedTech Forum 2025
𝗔𝗱𝘃𝗮𝗻𝗰𝗶𝗻𝗴 𝗘𝗮𝗿𝗹𝘆 𝗙𝗲𝗮𝘀𝗶𝗯𝗶𝗹𝗶𝘁𝘆 𝗦𝘁𝘂𝗱𝗶𝗲𝘀 𝗶𝗻 𝗘𝘂𝗿𝗼𝗽𝗲

The #MTF2025 event in Lisbon, Portugal was a significant milestone for the project, which aims to revolutionize Early Feasibility Studies (EFS) for medical devices in Europe.
𝗞𝗲𝘆 𝗧𝗮𝗸𝗲𝗮𝘄𝗮𝘆𝘀
- 𝗖𝗼𝗹𝗹𝗮𝗯𝗼𝗿𝗮𝘁𝗶𝘃𝗲 𝗘𝗳𝗳𝗼𝗿𝘁𝘀: The session highlighted the power of collaboration among industry, academia, healthcare, and patient associations. This cross-sectoral partnership is crucial for developing a harmonized EFS methodology.
- 𝗣𝗮𝘁𝗶𝗲𝗻𝘁-𝗖𝗲𝗻𝘁𝗿𝗶𝗰 𝗔𝗽𝗽𝗿𝗼𝗮𝗰𝗵: Integrating patient input ensures that medical devices are tailored to patient needs, enhancing their effectiveness and acceptance.
- 𝗖𝗹𝗲𝗮𝗿 𝗚𝘂𝗶𝗱𝗮𝗻𝗰𝗲: Providing a roadmap for EFS will streamline processes for innovative companies, hospitals, and competent HTA authorities, making Europe a more attractive location for medical device innovation.

𝗙𝘂𝘁𝘂𝗿𝗲 𝗜𝗺𝗽𝗹𝗶𝗰𝗮𝘁𝗶𝗼𝗻𝘀
- 𝗕𝗼𝗼𝘀𝘁𝗶𝗻𝗴 𝗖𝗼𝗺𝗽𝗲𝘁𝗶𝘁𝗶𝘃𝗲𝗻𝗲𝘀𝘀: A harmonized EFS methodology will enhance Europe’s competitiveness in the global medical device market.
- 𝗔𝗰𝗰𝗲𝗹𝗲𝗿𝗮𝘁𝗶𝗻𝗴 𝗜𝗻𝗻𝗼𝘃𝗮𝘁𝗶𝗼𝗻: Well-run EFS will speed up the development and patient access to innovative devices, ultimately improving healthcare outcomes.
- 𝗥𝗲𝗮𝗹-𝗪𝗼𝗿𝗹𝗱 𝗜𝗺𝗽𝗹𝗲𝗺𝗲𝗻𝘁𝗮𝘁𝗶𝗼𝗻: The involvement of all stakeholders, driven by a common objective, increases the likelihood of successful implementation in the real world.

Next Steps
- 𝗖𝗼𝗻𝘁𝗶𝗻𝘂𝗲𝗱 𝗖𝗼𝗹𝗹𝗮𝗯𝗼𝗿𝗮𝘁𝗶𝗼𝗻: We will continue to work closely with our partners to refine and implement the EFS methodology.
- 𝗘𝗻𝗴𝗮𝗴𝗲𝗺𝗲𝗻𝘁 𝗮𝗻𝗱 𝗙𝗲𝗲𝗱𝗯𝗮𝗰𝗸: Ongoing engagement with stakeholders will be essential to ensure the methodology meets the needs of all parties involved.
Thank you to the moderator and excellent speakers at #MTF2025 for their insightful contributions and for making complex issues accessible and engaging to a wider audience.



Moderator
Niklas Blomberg, Innovative Health Initiative (IHI)
Speakers
- Rosanna Tarricone, SDA Bocconi
- Alexandra Poulsson, Norwegian Institute of Public Health
- Laura Sampietro-Colom, Hospital Clínic de Barcelona
- Fanny van der Loo, Edwards Lifesciences Europe
- Yasemin Zeisl, European Patients’ Forum
Innovative Health Initiative (IHI) MedTech Europe EFPIA – European Federation of Pharmaceutical Industries and Associations COCIR EuropaBio – the European Association for Bioindustries Vaccines Europe
Call for Papers on Early Feasibility Studies for Medical Devices
Clinical Therapeutics is inviting submissions for a special issue on Early Feasibility Studies for Medical Devices. This issue aims to delve into the characteristics, patterns, and growth of EFS studies, comparing regulatory systems between the US and Europe, and evaluating the impact of the FDA EFS Program.
Key Aspects
- Focus: Initial clinical safety and performance of medical devices in early development.
- FDA EFS Program: Established in 2013 to foster innovation and early access for American patients.
- Topics: Success in attracting early clinical investigations, safety issues, patient benefits, and feedback mechanisms.
Case studies on technologies that reached the market post-EFS are welcomed, as well as submissions with a focus on initial clinical safety and performance of medical devices in early development.
𝗚𝘂𝗲𝘀𝘁 𝗘𝗱𝗶𝘁𝗼𝗿𝘀:
Giuditta Callea, Associate Professor and Carlo Federici, Research Fellow, from SDA Bocconi, and members of the HEU-EFS coordination team.
𝗦𝘂𝗯𝗺𝗶𝘀𝘀𝗶𝗼𝗻 𝗗𝗲𝗮𝗱𝗹𝗶𝗻𝗲: September 30, 2025
For more details, visit the Clinical Therapeutics journal page https://www.sciencedirect.com/special-issue/321847/early-feasibility-studies-for-medical-devices-goldmine-or-fool-s-gold.
Innovative Health Initiative (IHI) MedTech Europe EFPIA – European Federation of Pharmaceutical Industries and Associations COCIR EuropaBio – the European Association for Bioindustries Vaccines Europe
#EFS #HealthcareInnovation #MedicalResearch #EvidenceGeneration
World Health Day 2025

Celebrating World Health Day 2025: Embracing Healthy Beginnings for Hopeful Futures
Our commitment to healthcare innovation ensures that every individual has access to the medical advancements they need.
As we acknowledge the importance of April 7, #WorldHealthDay, we highlight the transformative impact of technology in innovating for better healthcare systems.
At HEU-EFS, a public-private partnership of 22 institutions, funded by the European Commission, our lifecycle approach to clinical evidence generation involves stakeholders from the outset, aligning medical innovation with safety and regulatory standards to foster a robust healthcare ecosystem.
The Early Feasibility Studies (EFS) initiative is a testament to our dedication to timely innovation. It’s not just about access to new technologies but ensuring these advancements are safe, effective, and enhance patient care.
Together, let’s celebrate the cause of health equity and uphold the right to health as a fundamental human right.
Join the cause for a healthier future for all!
HEU-EFS Interim Consortium Meeting
On Monday, April 7th, our Consortium partners, Advisory Board, and Patient Advisory Group held a productive interim meeting, focusing on the latest advancements from several work packages.
Key Highlights:
- Guest Speaker: We were delighted to welcome Danielle Giroud, who presented the latest updates to ISO Standard 14155.
- Valuable Input: The Advisory Board and Patient Advisory Group provided essential feedback on the importance of clear processes, stakeholder involvement at both EU and national levels, data collection, templates, and checklists.
- Next Steps: We outlined the next steps for the pilot use-cases, aiming to test and improve the future EU EFS Program.
A big thank you to all contributors for making this meeting valuable and productive. Together, we are laying a strong foundation for future success!
SDA Bocconi Fundació Clínic per a la Recerca Biomèdica Institut d’Investigacions Biomèdiques August Pi i Sunyer (IDIBAPS) Hospital Clínic de Barcelona Agenas Greater Paris University Hospitals – AP-HP Idris Oncology BV Meditrial Norwegian Institute of Public Health Trinity College Dublin The Philipp University of Marburg CIMT – Centre for Innovative Medical Technology Carmat Fondazione Policlinico Universitario Agostino Gemelli IRCCS European Patients’ Forum Newronika S.p.A. Global Heart Hub Qurasoft Edwards Lifesciences Europe Medtronic W. L. Gore & Associates Philips Abbott DePuy Synthes
Innovative Health Initiative (IHI) MedTech Europe EFPIA – European Federation of Pharmaceutical Industries and Associations COCIR EuropaBio – the European Association for Bioindustries Vaccines Europe
Work Package 3 Video Guide

We are pleased to share the progress being made in Work Package 3 of the HEU-EFS consortium, dedicated to enhancing early feasibility studies (EFS) for medical devices across Europe.
We invite you to watch the video to learn more about our work and its impact.
Alexandra Poulsson from the Norwegian Institute of Public Health and the Norwegian Medical Products Agency is leading efforts to develop comprehensive eligibility criteria for EFS. Her work ensures that technologies, medical conditions, patient severity, evidence levels, clinical sites, and expertise are all meticulously evaluated to pave the way for successful studies.
Nataliya Deych, Regulatory expert at Edwards Lifesciences Europe, emphasises the importance of collaboration between industry and regulators, highlighting the critical role of patient perspectives in shaping regulatory decisions. This commitment to innovation drives efforts to bring cutting-edge clinical programmes to European stakeholders.
Carlo Federici from SDA Bocconi contributes his expertise in health economics and technology assessments. Carlo’s research focuses on analysing the current state of pre-market clinical investigations and proposing a robust framework for EFS in the EU. His work is crucial for ensuring that medical devices are evaluated with the right evidence at the right time.
Visit heuefs.eu and follow us on LinkedIn https://lnkd.in/ekMx-4mp and X https://twitter.com/HEUEFS as we advance towards a harmonised approach to early feasibility studies, ultimately enhancing patient access to innovative medical technologies.
Innovative Health Initiative (IHI) MedTech Europe EFPIA – European Federation of Pharmaceutical Industries and Associations COCIR EuropaBio – the European Association for Bioindustries Vaccines Europe
#IHITransformingHealth #PatientCare #Evidence #MedTech