Deliverables
Annual Consortium Meeting in Geneva

[7-8 October 2024, Geneva] – The HEU-EFS Consortium gathered for our annual consortium meeting, marking a significant milestone in our journey towards harmonizing Early Feasibility Studies (EFS) across Europe.
Meeting Recap:
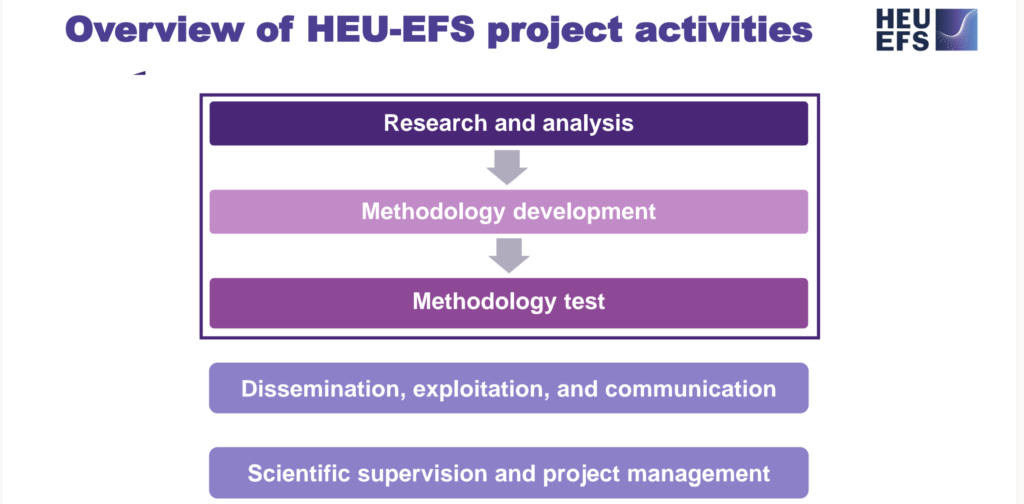
- Research and analysis on the state of play of premarket programs and implementation barriers to EFS (WP1) & regulatory framework and institutional and organizational characteristics of EU competent authorities (WP2)
- The initial results of the research-focused work packages have been shared and discussed with the HEU-EFS advisory bodies, including the Patient Advisory Group and the Advisory Board. Their feedback is crucial to ensure that both WP1 and WP2 provide a solid foundation for methodology development activities.
- In reviewing the premarket programs, the University of Bocconi has designed a survey for MedTech innovators, including companies, SMEs, and start-ups involved in medical device development and clinical investigations. The survey aims to gather their experiences and challenges in pre-market clinical investigations. Take the survey here.
- Click here to watch what WP1 and WP2 are focusing on, as described by our Work Package leaders.
- Research and analysis on the regulatory framework and institutional and organizational characteristics of EU competent authorities (WP2)
- Engaged in interactive sessions with the Patient Advisory Group (PAG) and Advisory Board (AB) to gather valuable feedback.
- Methodology development: evidence requirements, data, and statistical tools (WP3)
- The orchestration of methodology development activities is now the primary focus of the HEU-EFS project. To establish a widely accepted framework, HEU-EFS ensures the inclusion of all key stakeholders in the methodology development process.

The collaboration and expertise of our consortium members were on full display as we worked together to address the challenges and opportunities within the EFS ecosystem. We are excited to share that our collective efforts are paving the way for a more efficient and effective European Health System.
Online Survey for Sponsors of Medical Device Clinical Investigations

Introduction
Sponsors of pre-market clinical investigations for medical devices are invited to participate in an online survey conducted as part of the Harmonised Approach to Early Feasibility Studies for Medical Devices in the European Union (HEU-EFS) project (Grant Agreement no. 101112185).
Funded by the Innovative Health Initiative (IHI), HEU-EFS aims to develop a robust, standardized methodology and set recommendations to streamline Early Feasibility Studies (EFS) within the EU.
Establishing a clear, harmonized framework for EFS in the EU is expected to benefit patient access, innovation uptake and investment attractiveness in Europe.
Survey
This survey focuses on pilot pre-market clinical investigations (CI) to assess a medical device early in its development phase. These include first-in-human, early feasibility, and traditional feasibility clinical investigations. When answering, please consider specifically the challenges of planning, monitoring and conducting CI in Europe.
Take the survey https://bit.ly/HEU-EFS_Survey
The survey will be open until 11 November.
The results will be presented in a dedicated webinar and on the HEU-EFS project webpage.
ISPOR Europe 2024

[17-20 November 2024 | Barcelona, Spain] Representing the HEU-EFS project, partners from SDA Bocconi and Trinity College Dublin will present a series of poster sessions.
Subject of Debate
Innovative, high-risk medical devices can revolutionize treatment but often require numerous modifications before arriving at a final design. Addressing both the important risks and significant benefits for patients, research centres, regulatory bodies, and sponsors is crucial. The main objective of the 4-year HEU-EFS Project, funded by the Innovation Health Initiative and involving 22 public and private partners, is to develop a harmonized methodology for the uptake of Early Feasibility Studies (EFS) in the EU that mitigates risks and reinforces benefits for various stakeholders.
Poster Sessions and Presenters:
- Global Assessment of Pre-Market Approval Pathways for Medical Devices: Highlighting the Need for Harmonization across 55 Jurisdictions
Session Time: 18 November 4:00 PM – 7:00 PM – HPR67
Speaker: Francesco Malandrini from SDA Bocconi - Use of Early Feasibility Studies to Inform Development of Medical Devices
Session Time: 20 November 9:00 AM – 11:30 AM – MT58
Speakers: Franco Luigi Zurlo from SDA Bocconi - The EU Regulatory Framework for Medical Device Early Feasibility Studies: What Do We Know to Date?
Session Time: 18 November 4:00 PM – 7:00 PM – HPR98
Speaker: Majella Geraghty from Trinity College Dublin

Who Should Attend:
These poster sessions can provide valuable insights for a diverse group of stakeholders, including EU-wide and national regulators, competent authorities, notified bodies, HTA bodies, healthcare providers, patient organizations, research organizations, legal and ethical experts, CROs, and health technology developers.
Work Package 2 Video Guide
In this video, you will hear from Tom Melvin (Trinity College Dublin) and Nicolas Martelli (Hôpital Européen Georges-Pompidou) as they elucidate that Work Package 2 of the HEU-EFS project is dedicated to ensuring the future EU EFS Program aligns with existing regulations and standards, and involves evaluating the readiness of EU competent authorities to support robust pre-market clinical investigations, particularly for Digital Health Technologies.
At HEU-EFS, we believe that collectively, as a consortium of 22 institutions, SMEs and private companies, we can impact the health and well-being of people in Europe and beyond.
Work Package 1 Video Guide

In this video, you will hear from Giuditta Callea (SDA Bocconi) and Marta Kerstan (DePuy Synthes) as they explain the comprehensive research and analysis we employ as a consortium to understand Early Feasibility Studies (EFS) and their regulatory environment, ultimately aiming to develop a harmonised EU methodology that will enhance their adoption and support medical innovation in Europe.
At HEU-EFS, we believe that collectively, as a consortium of 22 institutions, SMEs and private companies, we can impact the health and well-being of people in Europe and beyond.
HEU-EFS Interim Consortium Meeting
Advancing Premarket Programmes and Communication

[10 April 2024, online] – The HEU-EFS Consortium convened an online interim review meeting, marking a significant milestone in our journey towards harmonising Early Feasibility Studies (EFS) across Europe.
Key Outcomes:
- Conducted detailed research of the regulatory framework and premarket programmes, pinpointing challenges to the implementation of Early Feasibility Studies (EFS).
- Strategies are being developed to address these challenges, aiming to streamline the process for healthcare innovations.
- The website is evolving into a key resource for disseminating research findings and engaging the healthcare community.
- Communication enhancements are underway to build a network of stakeholders vital for the EFS implementation across the EU.
- Tools for monitoring the performance of the EFS programme are in the development phase.
The collaboration and expertise of our consortium members were on full display as we worked together to address the challenges and opportunities within the EFS ecosystem. We are excited to share that our collective efforts are paving the way for a more efficient and effective European Health System.

A Video Guide to the HEU-EFS Project
In this video, you will hear from Rosanna Tarricone (SDA Bocconi) and Andrea Rappagliosi (Edwards Lifesciences Europe) as they explain the challenges and opportunities of medical device innovation in the European Union. You will also learn more about the HEU-EFS project’s objectives, activities, and expected impact.
At HEU-EFS, we believe that collectively, as a consortium of 22 institutions, SMEs and private companies, we can impact the health and well-being of people in Europe and beyond.
Call for interest: Applications welcome for HEU-EFS’s Patient Advisory Group
Consortium partners are pleased to announce a call for representatives for a Patient Advisory Group (PAG) for the project ‘Harmonised Approach to Early Feasibility Studies for Medical Devices in the European Union (HEU-EFS)’. Applications remain open until 20 February 2024.
The Patient Advisory Group will discuss and provide recommendations for structured patient contribution to Early Feasibility Studies, in short EFS, to make more patient-centred medical devices. Early Feasibility Studies (EFS) are small-scale research studies or tests done in the very early stages of developing a medical device or treatment to see if it is practical, safe, and worth pursuing further. EFS help assess whether an idea or concept has potential before investing more time and resources into full-scale development and testing. The patient perspective in Early Feasibility Studies is highly important.
The PAG is meant to form a hub for patient centricity and engagement across HEU-EFS’s implementation. The aim is to recruit a maximum of ten representatives. Read more here

