Open Call For Pilots
Early Feasibility Studies in European Union
ISO 14155:2020 defines Early Feasibility Studies (EFS) as limited clinical investigations designed to evaluate the initial clinical safety and performance of a medical device.
Conducted at an early stage, before the device design is finalised, EFS help collect critical insights on a device’s design, safety, functionality, and performance, especially when further nonclinical testing is unavailable or not informative.
Why EFS Matter
EFS play a key role in the evidence generation pathway, offering the potential to:
- Increase investment attractiveness in the European medical technology landscape
- Accelerate patient access to safe and innovative medical devices.
- Strengthen the EU’s position as a hub of clinical excellence and innovation.
The Current Challenge
While EFS are permitted under the EU Medical Device Regulation (MDR), there is currently no harmonised pathway or specific framework to support their systematic adoption across the European Union (EU).
The HEU-EFS initiative aims to change this by developing a unified, structured approach to Early Feasibility Studies, enabling innovation and collaboration across Europe.
The HEU-EFS project
HEU-EFS is a four-year European project called Harmonised Approach to Early Feasibility Studies for Medical Devices in the European Union, which started in October 2023 and involves 22 public and private organisations across Europe.
The project is co-funded by the Innovative Health Initiative (IHI) under the Horizon Europe Framework Programme and 6 industry partners for a total budget of €19 million. The overall goal of the initiative is to develop a harmonised methodology and enhance the uptake of EFS in the EU. For more information, please visit www.heuefs.eu.
Call for expression of interest
HEU-EFS consortium is pleased to announce the launch of an open call for pilots to validate the methodological framework of the harmonised approach to EFS for medical devices in the EU.
As a first step, under this call, pilot proposers are invited to submit Expression of Interest online form to indicate their interest to conduct an EFS pilot study using methodology developed by the HEU-EFS consortium.
The pilot will provide innovators with a unique opportunity to take their innovations via a pilot structured EFS regulatory process. Particularly, the participants will:
- Obtain guidelines and templates for EFS submissions along with structured early engagement with the National Competent Authorities (NCAs).
- Benefit from a unique learning opportunity and engage with our consortium experts.
- Contribute feedback that will influence the development of the future EU EFS programme.
Who can apply and eligibility conditions
Sponsors wishing to conduct pilot EFS clinical investigations in Europe who meet the following criteria:
1. The clinical investigation must be consistent with the definition of Early Feasibility Study as per ISO 14155:2020.1
2. They must be a high-risk devices (Class III and Class IIb), where a clinical investigation will be required as part of the conformity assessment.
3. They must meet at least one of the following criteria:
- Breakthrough Device/Unmet Need – utilized to collect early clinical data when no equivalent device exists.
- Anatomical understanding – utilisation of the EFS to better understand physiology and anatomical limitations of the device under development.
- New /Expanded intended uses or indication for use – the application of an existing/approved device in a new patient population or application
- They must request approval in at least one European country (i.e. countries within the EU or European Economic Area).
Submission of the call of interest
Applications should be submitted by completing and sending the online form no later than March 31, 2026.
The data provided will be handled as strictly confidential. It will be stored by Bocconi University (project coordinator) and accessible to members of the Screening Committee.
For any questions regarding the Open call for pilots, please contact the HEU-EFS Point of Contact at pilot@heuefs.eu.
Process
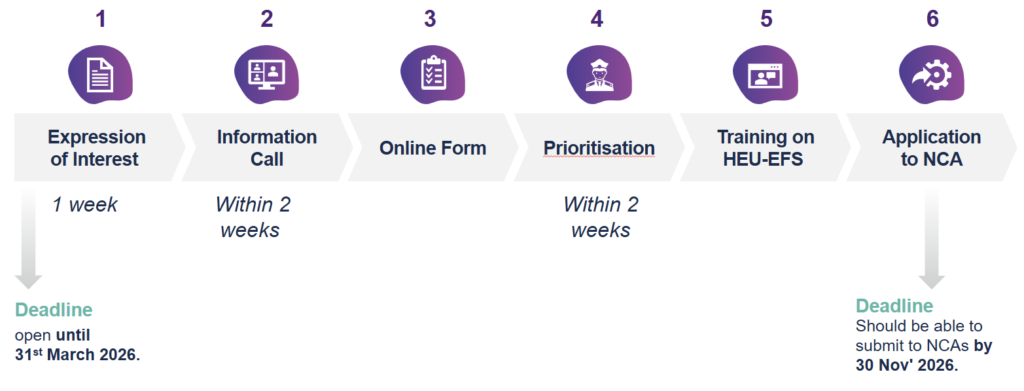
The Expressions of Interest will be assessed by the Screening Committee, coordinated by Bocconi University to review matching of the pilots with the scope of the methodology framework and proposer’s readiness.
Proposers and members of the Screening Committee will meet online (30-minute) and discuss the expressed interest.
After positive assessment, proposers will be provided with the list of next steps and with a link for submitting a self-evaluation checklist via the online system QualtricsXM provided by Bocconi University.
Once an applicant is selected for the pilot, the Point of Contact will make available an information package and training outlining the next steps and providing instructions and templates. No services will be provided on the conduct of the clinical trial implementation. Proposers should finance their own studies. No public funding will be available from Innovative Health Initiative or from HEU-EFS consortium.
Want to revisit the highlights from our Public Forum on Early Feasibility Studies or highlights from Pilot Programme webinar?
You can now watch the full recording below and download the presentations shared during the event:
Resources
Find all project deliverables here.